Part:BBa_K2819103
Blue Light Activated Repressible Promoter with RFP Reporter attached to YbaQ Degradation Tag
This part contains a promoter that can be repressed by blue light (450nm). This promoter makes use of a blue light dependent DNA-binding protein, EL222. Irradiation by blue light of wavelength 450nm exposes the hitherto sequestered HTH, facilitating dimerization of EL222 and subsequent DNA binding. The repression is achieved by placing the DNA binding site of EL222 between the -35 and -10 hexamers of the consensus promoter in E. coli, creating the blue light repressible promoter PBLrep. As a result, EL222 acts as a repressor, blocking the binding of RNA polymerase and repress gene expression in the presence of blue light. In the dark, RNA polymerase can now bind, and gene expression takes place.
Biology
Originating from the marine bacterium Erythrobacter litoralis HTCC2594, EL222 is a photosensitive DNA binding protein, with a N-terminal light-oxygen-voltage (LOV) domain and a C-terminal helix-turn-helix (HTH) DNA binding domain.
Improvement over existing part BBa_K2332021 by iGEM17_UCL
We aim to use this new part to improve the existing composite part. We hope to understand the dynamic characteristics of the blue light repressible promoter (PBLrep) better, so that we can use it to the application in our project. While the existing part has a bioreporter, the coding region of the bioreporter, LuxCDABE, has a large gene cluster (>5500bp), making it difficult for dynamic characterization due to its complexity. Furthermore, there is no characterisation data available on iGEM17_UCL’s part registry page for the blue light repressible promoter BBa_K2332019. Therefore, our team sought out to conduct a study on the PBLrep, by using a more suitable reporter. We altered the sequence of this composite part by replacing the LuxCDABE coding region with a commonly used reporter gene, red fluorescent protein (RFP, ~700bp), as well as tagging different degradation tags to the reporter gene to study the characteristics of this promoter. Furthermore, this new part has now wider applications compared to the previous part, such as an indicator. As the overall plasmid size is reduced significantly, it is now easier for users to co-transform it with other plasmids.
Characterization
To characterize this promoter, we used a common reporter gene, RFP (Red Fluorescence Protein). However, we quickly found that the expression of RFP cannot be regulated by this promoter, as its expression profile did not follow induction patterns. Therefore, we altered the sequence of the coding region to obtain a degradation rate optimal for this promoter to work, improving its functionality as now users can have better knowledge on how to use this promoter. To achieve this, we attached RFP to different degradation tags. We did our characterization using various blue light on-off patterns. The PBLrep can be regarded as a constitutive promoter when blue light is absent, i.e. in dark, as dimerization of EL222 will not take place.
Methods
All constructions and characterizations were done in E. coli TOP10, all plasmids contain kanamycin resistance. 3 composite parts were used: PBLrep-RFP (original part), PBLrep-RFP-DAS and PBLrep-RFP-YbaQ. All 3 were tested simultaneously in each experiment. Engineered cells were inoculated in 5mL of LB with kanamycin overnight. The next day, 5uL of cell culture was refreshed in 5mL of fresh LB with kanamycin until its OD reaches 0.05. At OD600 = 0.05, 1mL of cell culture was transferred to each well of a 12-well plate. Cells were then incubated in 37°C at 220 rpm, under different light conditions depending on the experimental setup. Fluorescence of the cells was measured at 1-hour intervals using BioTek Synergy H1 microplate reader.
Cells were characterised in 12-well-plates in this experiment, and each sample is measured in triplicates. An example of a setup is as shown below.
‘DAS’ represents PBLrep-RFP with DAS degradation tag; ‘YbaQ’ represents PBLrep-RFP with YbaQ degradation tag
Characterizing under Blue Light
Persistent Light On and Light Off
In this experiment, one setup was incubated under persistent blue light for 8 hours, while another setup was incubated in the dark (covered with black cloth) for the same duration.
Our data suggests that using RFP solely as a reporter is unable to reflect the expected induction and repression activities of the promoter. As seen from the Figures 3a and 3b, fluorescence decreased from 0 to 4 hours in dark, and increased from 4 to 8 hours under blue light, which are completely opposite of what we expected. We speculated that this phenomenon to be due to the mismatch in rate of increase in OD600 and the degradation rate of RFP. Hence, to alter the degradation rate of RFP, we attached 2 different degradation tags, YbaQ and DAS. Degradation tags are also referred to as degrons. They are either part of coding regions, or are added to them to direct their degradation by proteases.
Experimental results show that RFP attached to YbaQ could reflect the expected induction and repression activities of PBLrep. As shown in Figure 3a, RFP per OD increased steadily with time in dark, where repression is absent, while RFP/OD decreased exponentially with time under blue light as shown in Figure 3b. The high initial RFP/OD at 0 hour in Figure 3a can be explained by the low initial OD600 (0.05) and the presence of RFP in the system before the experiment commences.
[Formula: RFP/OD_light off ÷ RFP/OD_light on]
Numbers on top of bar graphs represent the fold change
Figure 4a and 4b represent the comparison of RFP/OD of persistent light on and off conditions for PBLrep-RFP and PBLrep-RFP-YbaQ. There is a clear increasing trend for fold change for PBLrep-RFP-YbaQ, while fold change for PBLrep-RFP increased from 0 to 4 hour, then dropped from 4 to 8 hour.
This characterisation experiment indicate to us that the degradation rate of RFP-YbaQ is required for this promoter to work as intended.
Varying Blue Light On-Off Patterns
To verify that the degradation rate of RFP-YbaQ is indeed the most optimal rate for PBLrep to work, we carried out further characterisation experiments with different blue light on-off patterns.
As observed from the graphs, even under different light on-off patterns, PBLrep-RFP-YbaQ still performed the best, showing clearer oscillation patterns with the absence and presence of light, while PBLrep with just RFP alone or with RFP-DAS showed relatively poor responses with the rate which light is being switch on and off.
Modelling PBLrep and Verification of Model
We also modelled the activity of the promoter and our model shows that for PBLrep to reflect better oscillation patterns, we should incubate the cell cultures in dark for 45 minutes, and under blue light for the remaining duration of the experiment.
Our wetlab experimental data verified the recommendations made by our model. A dip is no longer observed at the start of the graph, and the change in RFP/OD could reflect our light on-off pattern.
Conclusion
Our improvement in change the coding region sequence of a common reporter gene, RFP, by adding an additional sequence of a degradation tag, YbaQ, has allowed us to better understand how we can use this the blue light repressible promoter. From our characterization results, we therefore recommend future users to use a coding region with the same or similar degradation rate as RFP-YbaQ, in order to achieve the desired function of this promoter. This new part has hence improved the functionality of this promoter, which has helped us to be mindful when we select the genes we use under this promoter in our project.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal XhoI site found at 551
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 121
Illegal AgeI site found at 1425
Illegal AgeI site found at 1537 - 1000COMPATIBLE WITH RFC[1000]
Group
NUS_Singapore 2019
Summary and Uploads
Improvement over existing part BBa_K2819103 by iGEM18 NUS Singapore-A
While many teams have previously characterized blue-light repressible systems, there are not many investigations done on the potential effect of spacer DNA behind the promoter on protein expression. The part BBa K2819103 by iGEM18_NUS Singapore-A had an 11 base pair long spacer DNA behind the blue-light repressible promoter. This year, we constructed two different new plasmids. One of them had an increased spacer DNA sequence with 30 base pairs, while the other one had the spacer sequence removed completely. Our aim was to investigate how the length of this spacer DNA would affect the performance of the blue-light repressible system. Our hypothesis is adding a longer spacer sequence would increase the RFP expression while removing the spacer sequence would decrease the RFP expression.
Characterization
The three types of plasmid were transformed into Escherichia coli MG1655 strain for characterization. For simplicity, the three types of cells are referred to as 0bp, 11bp and 30bp. 50uL of overnight culture of 0bp 11bp and 30 bp MG1655 cells were transferred into 5mL LB+Chloramphenicol medium in three 50mL tubes. They were then refreshed to a starting culture and incubated in 37 °C for one and a half hours until OD600 reaches about 0.8 to start characterization.
12 well plates were used for characterization. The plate layouts are the same for both plates.
Plate layout: first column: triplicate of 0bp MG1655, second column: triplicate of 11bp MG1655, third column: triplicate of 30bp MG1655, last column, triplicate of blank LB medium (Figure 1).


Figure 1: Plate layout for characterization of improved part
1mL of each cell and medium culture were transferred into each well in the 12 well plates. Initial OD600 and RFP readings were taken at 0h time point using H1 Synergy microplate reader. The protocol is shaking for 10s, reading OD600, and reading RFP. The results are exported as excel. For subsequent readings, the protocol is the same throughout and all results are exported in the excel sheet.
After the first reading, 1 plate was placed on the blue light device to be exposed to blue light, while the other plate is covered with a black cloth to prevent any exposure to light (Figure 2). Both are incubated in a shaking incubator at 37 °C and shaking at a speed of 125rpm. Hourly readings were performed for the next 8h.

Figure 2: Experimental setup for blue light and dark conditions
Results
The following figures (3 and 4) are the growth curves of cells with and without blue light exposure.
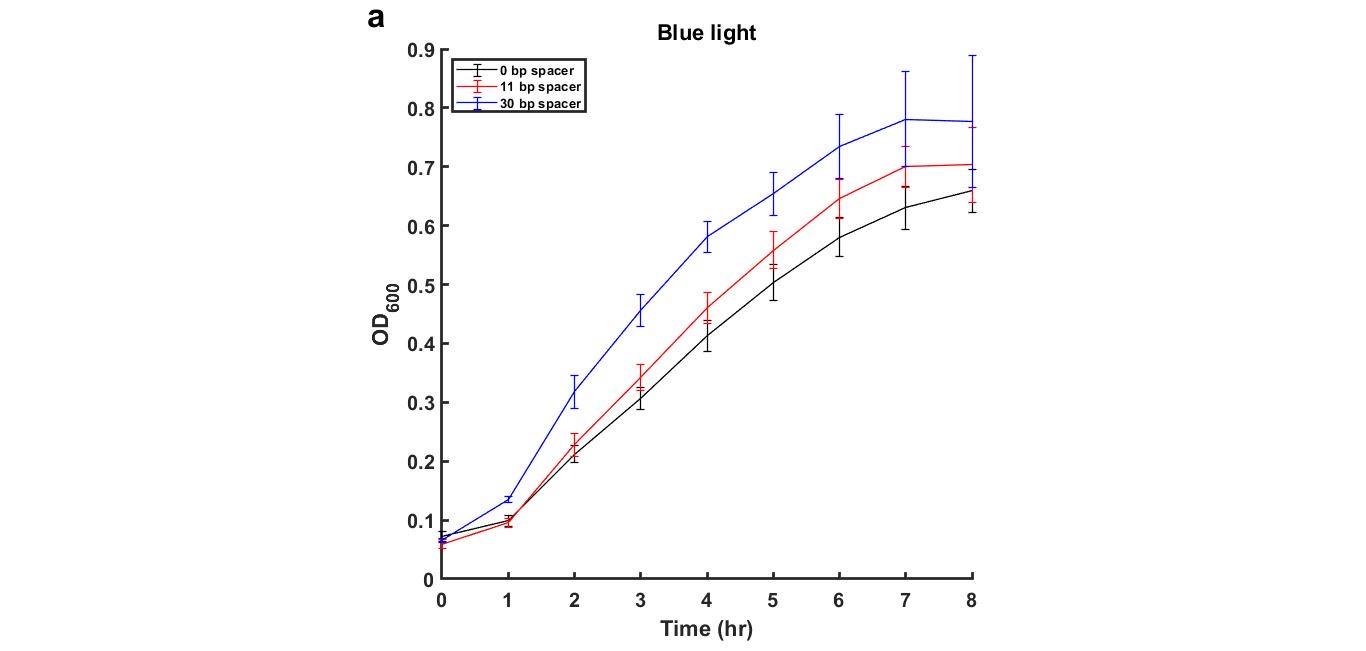
Figure 3: Growth curves of MG1655 transformed with plasmid containing blue-light repressible promoter and different length of spacer DNA under blue light environment

Figure 4: Growth curves of MG1655 transformed with plasmid containing blue-light repressible promoter and different length of spacer DNA under dark condition
Generally, there is no significant difference between the OD600 curves of cells in the dark and blue light condition. However, we noticed that under both Dark and Blue light conditions, the cells with a plasmid containing 30 base pair spacer DNA sequence grew faster and reached a higher final OD600 as compared to 11bp and 0bp.
The following figures (5 and 6) illustrates the production of RFP in the dark and under exposure to blue light.

Figure 5: RFP production curves of MG1655 transformed with plasmid containing blue-light repressible promoter and different length of spacer DNA under blue light environment

Figure 6: RFP production curves of MG1655 transformed with plasmid containing blue-light repressible promoter and different length of spacer DNA under dark condition
We observed that the blue-light repressible system was working well. For cells incubated in the blue light environment, we could see that their RFP production decreased significantly over time while the cells incubated in the dark had an increasing RFP production. It is clear that cells containing the plasmid with 30 base pair spacer DNA had the highest RFP production overtime. The RFP production of this new construct is 3.8 fold as compared to the original construct (11 base pair). However, the cells with their spacer DNA completely removed (0 base pair) did not seem to produce RFP at all. Therefore, we can conclude that increasing the spacer DNA length behind the blue-light repressible promoter increases the RFP production while decreasing the spacer DNA reduces RFP production.
Combining the findings for OD600 and RFP, we had an interesting observation: The cells producing higher amount of RFP (longer spacer length) also grew faster and reached a higher final OD600. This is different from what we expected. We assumed that cells producing higher amount of RFP would have a greater metabolic burden and therefore grow slower.
The following figures (7 and 8) illustrate the ratio of RFP to OD600.

Figure 7: RFP production per OD600 curves of MG1655 transformed with plasmid containing blue-light repressible promoter and different length of spacer DNA under blue light environment

Figure 8: RFP production per OD600 curves of MG1655 transformed with plasmid containing blue-light repressible promoter and different length of spacer DNA under dark condition
Taking into account of cell density, we also plotted the RFP/OD600 curve. Our conclusion is clearly supported by these curves as well, with a longer spacer DNA sequence demonstrating a much larger fold change between production and repression of proteins. This therefore indicates that a larger spacer sequence would result in a more effective blue-light repressible system.
References
Takakado, A., Nakasone, Y., & Terazima, M. (2017). Photoinduced dimerization of a photosensory DNA-binding protein EL222 and its LOV domain. Physical Chemistry Chemical Physics, 19(36), 24855-24865.
Source
BBa_K2819103 originated from Erythrobacter litoralis obtained from iGEM18_NUS_Singapore-A.
Design Considerations
Generally, the basal level of protein production under blue-light repressible promoter is low. In order to better characterize the promoter and clearly show the repression, we wanted to achieve higher basal protein production. We hypothesized that a longer spacer sequence between the promoter and coding region might allow easier binding of the transcription factors and faster formation of transcription initiation complex which might lead to higher protein production. We also carefully designed the sequence of the spacer DNA such that it does not interfere with other parts of the gene, especially the coding sequence.
Link to our Improved Part
| None |









